Munenori NUMATA
(Associate professor, PI)
(CV, Personal Link)
Chisako KANZAKI (D1)
Shota MATOBA (M1)
Hiroshi YONEDA (M1)
Haruna TAKEMORI (B4)
Kae YAMASHITA (B4)
Misaki YAMAJI (B4)
Munenori NUMATA
(Associate professor, PI)
(CV, Personal Link)
Chisako KANZAKI (D1)
Shota MATOBA (M1)
Hiroshi YONEDA (M1)
Haruna TAKEMORI (B4)
Kae YAMASHITA (B4)
Misaki YAMAJI (B4)

Labo Member
Research Out-line
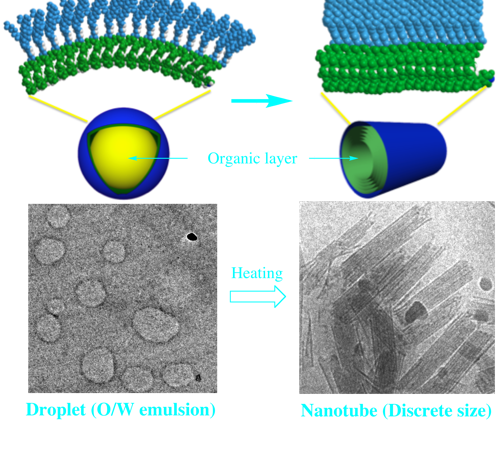
A novel approach toward discrete sub-micrometer-sized one-dimensional structures
Developing a new field of molecular self-assembly in the sub-micrometer regime—with precision as high as that used to make discrete nano-sized molecular architectures through molecular programming—is a major challenge for supramolecular chemistry. At present, it is impossible to create discrete sub-micrometer-sized molecular architectures only through conventional supramolecular systems based on molecular programs, because there is no effective strategy for controlling the assembling molecules when there are more than a thousand of them (for example, 1-D structures such as rods and tubes typically undergo uncontrollable growth).
In our developed system, carefully designed amphiphilic molecules are compartmentalized into droplets with narrow distributions of their sizes and molecular number; these droplets are subsequently transformed into discrete tubular structures having dimensions on the sub-micrometer scale. The lengths and multiplicities of tube layers (single- or multi-wall) can be tuned merely by changing the droplet’s properties in a top-down manner.
Contact:
Department of Biomolecular Chemistry, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Shimogamo, Sakyo-ku, Kyoto 606-8522, Japan
E-mail: numata at kpu.ac.jp
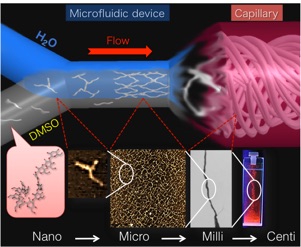
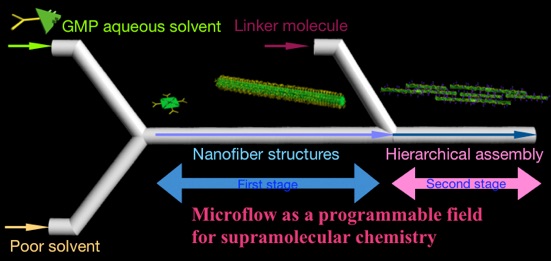
Constructing a versatile self-assembly system will lead to technological innovations in supramolecular chemistry. This would resemble the development of a new reaction or a new catalyst in synthetic organic chemistry. We propose a novel, practical supramolecular assembly system in combination with microflow techniques that enable control of intermolecular or interpolymer interactions through precise regulation of a flowing self-assembly field. The potential of microflow was explored by utilizing various simple model compounds. We found that rapid activation of molecules under a non-equilibrium state caused by uniform solvent diffusion drastically enhanced interactions in microflow. All self-assembly processes started from a temporally activated state and proceeded in a uniform chemical environment, forming a synchronized cluster and resulting in quantitative conversion to supramolecules, with tuning of molecular interaction and long-range regulation over a molecular scale. Thus, the developed system is completely different from conventional self-assembly occurring in a bulk solution. This leads to the synthesis of a variety of novel supramolecules such as discrete microstructures, hierarchical assemblies, and supramolecules with time-delayed action, which were previously inaccessible.
Tuning intermolecular interactions in microfluidic device:
toward supramolecular flow chemistry
Supramolecular polymerization of pi-conjugated molecules is an attractive way to create functional materials by amplifying the inherent molecular functionality. To date, the self-assembly and resultant material properties of pi-conjugated molecules have been governed primarily through molecular design. In contrast, little attention has been paid to positive control of the self-assembly process itself—especially the rate of self-assembly, a factor that strongly affects the size, homogeneity, and functionality of the resultant supramolecular polymers. We believe that, in addition to molecular design, precise control over the self-assembling field would be indispensable for the preparation of functional supramolecular polymers. At present, however, there are no versatile strategies for approaching this problem; therefore, any comprehensive means of overcoming this limitation of supramolecular chemistry would be highly attractive and applicable for tuning the functions of molecular pi-electron systems.
In our developed system, the microflow provides a practical self-assembling field for controlling intermolecular interactions of various pi-conjugated molecules. For example, we have found that the rate of nucleation could be controlled merely by changing the flow rate—i.e., the system’s hydrodynamic properties, rather than the chemical environment used in the conventional systems. Indeed, increasing the flow rate activated molecules toward self-assembly, with the stabilities of supramolecules being enhanced remarkably, accompanied by dramatically extended lengths. This finding suggests that we can select the pathway for self-assembly merely by tuning the hydrodynamic properties of a solution in a top-down manner.
How to control different intermolecular interactions
Despite self-assembly events being strongly affected by the environments surrounding the molecules and by the timing of the molecules interacting with one another, relatively little attention has been paid to positive control over the field of self-assembly. A comprehensive strategy for overcoming this limitation of supramolecular chemistry would be highly attractive and applicable. We have proposed a potential solution by exploiting a novel supramolecular system in conjunction with microfluidics, enabling us to precisely control the sequence of intermolecular interactions under non-equilibrium conditions, thereby leading to the creation of hierarchical molecular architectures with various complexities.
M. Numata, T. Kozawa, Chem. Eur. J., 20, 6234-6240 (2014) (Inside Cover).
M. Numata, T. Kozawa, R. Nogami, K. Tanaka, Y. Sanada, K. Sakurai, Bull. Chem. Soc. Jpn. 88, 471-479 (2015).
M. Numata, T. Kozawa, T. Nakadozono, Y. Sanada, K. Sakurai, Chem. Lett. 44, 577-579 (2015) (Editor’s Choice).
M. Numata, D. Kinoshita, N. Taniguchi, H. Tamiaki, A. Ohta, Angew. Chem. Int. Ed., 51, 1844-1848 (2012).
M. Numata, Y. Takigami, N. Hirose, R. Sakai, Org. Biomol. Chem., 12,1627-1632 (2014).
M. Numata, Y. Takigami, M. Takayama, Chem. Lett., 40, 102-103 (2011).
M. Numata, Y. Takigami, M. Takayama, T. Kozawa, N. Hirose, Chem. Eur. J., 18,13008-13017 (2012) (VIP Paper).
M. Numata, T. Kozawa, Chem. Eur. J., 19, 12629-12634 (2013).
M. Numata, R. Sakai, Chem.Lett., 43, 577-579 (2014).
M. Numata, R. Sakai, Bull.Chem.Soc.Jpn.,87, 858-862 (2014) (BCSJ Award Article).
I.Numata, Y. Nishino, Y. Sanada, K. Sakurai, Chem. Lett., 44, 861-863 (2015).
In conventional supramolecular strategies, molecular events are always treated as averaged states. If systems are regulated under equilibrium, this situation poses no problems even in the synthesis of fine supramolecules. On the other hand, when Avogadro’s number of molecules self-assemble in bulk under kinetic control accompanying a phase separation, the self-assembly field is no longer uniform at the molecular level, leading to a different self-assembly process depending on the spatial point. Little attention has been paid to this general drawback in supramolecular chemistry.
A straightforward way to achieve exact molecular control is dividing a bulk solution into picoliter-volume fragments. This approach has several advantages, including enhanced interactions, controlled molecular packing, long-range regularity, and amplification of nanostructures, which comprise a technical innovation in chemistry.
What is a new paradigm of self-assembly?
A potential candidate; Flowing micro-space
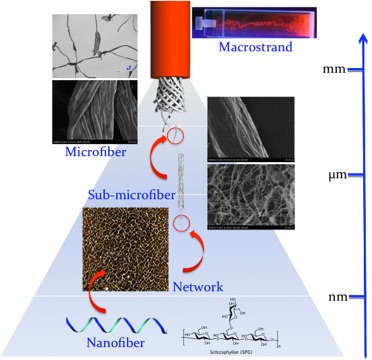
Tuning polymeric nanostructures in microfluidic device
As a feature aspect of nature’s self-assembling system, the creation of the micrometer-sized structures is dynamically controlled depending on the physiological environment, which compensates for the conspicuous self-assembling abilities of each component protein; some proteins have the potential to switch their self-assembling ability, which is generally driven by chemical fuel such as adenosine triphosphate (ATP), depending on the physiological environment. For example, spider silk protein features this unique ability; it is stored in its stable form without forming any aggregate, but spontaneously transforms into reactive form on demand, creating stable microfibers.
The laminar flow enables chemists to precisely control local concentrations of polymers, mixing rate of solvents and shearing force applying polymer chains, which can switch their inherent self-assembling nature of self-assembling polymers.
The application of microflow to control self-assembly provides the following advantages;
(1) This enables precise kinetic control by coupling rapid and uniform diffusion of solvents, which results in a reliable and reproducible kinetic self-assembly process.
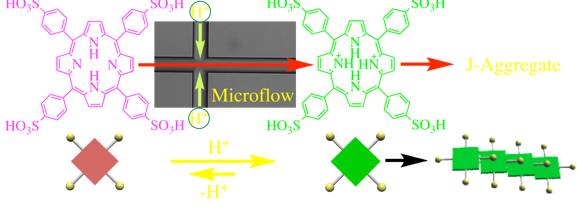
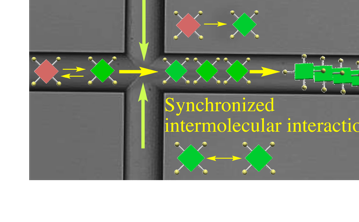
(2)Shift from chemistry of individual molecules to chemistry of a molecular cluster. In principle, all self-assembly events start simultaneously and finish at the same spatial points; that is, all processes starting with molecules and forming supramolecules and their hierarchical assemblies can be synchronized over a molecular scale. This leads to a marked enhancement of intermolecular interactions, simultaneous self-assembly of more than thousands of molecules towards monodisperse microstructures, and quantitative conversion from molecules to supramolecules.
(3) Direct correlation between self-assembly dynamics and flow distance. In principle, self-assembly dynamics can be controlled along a channel. In the case of diverse self-assemblies, such as supramolecular polymers, the self-assembly number (molecular weight) can be regulated by the flow distance. Similarly, molecular complexity combining different molecules and hierarchies appear with time progress along the channel.
(4) Selectable kinetic pathway towards the desired functionalities. Aggregation rates may be precisely tuned through flow-field programming – diffusion time of solvent across the channel and gradation of solvent polarity along the channel. Therefore, intermolecular interactions are tunable and afford various supramolecular functionalities such as J and H aggregates from a single molecular component.
(5) Versatile self-assembly platform independent of the molecular nature. The concept of self-assembly based on molecular design is still practicable with microflow, but it has less of an effect on the final structure. Balancing molecular design and flow-field programs governs the final structures of self-assemblies.
(6)Energy expenditure of assembly. In microflow, self-assembly always occurs during uniform solvent diffusion (across the stream) and during laminar flow (along the stream). Self-assembly is compensated for solvent diffusion that generates entropy (dS/dt > 0). In principle, energetically uphill processes are allowed.
Time-delayed action of self-assembling molecules
It is widely accepted that cooperative intermolecular interactions play an important role in the formation of fine, self-assembled architectures. However, this concept applies only to thermodynamic conditions. Our latest results using OPV suggest that under microflow with kinetic control, only a favorable interaction can be predominate, depending on the applied self-assembly. Since the resultant self-assembled structures are metastable, decomposition toward thermodynamically stabilized structures occurs spontaneously. In general, microflow provides “open environments” supplied by external energy. Even an unfavorable self-assembly process can be driven by energy.
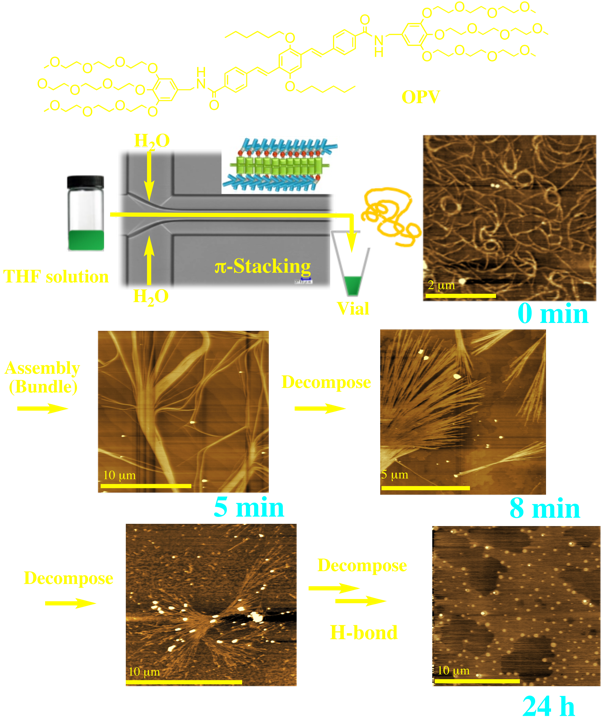
M. Numata, A. Sato, R. Nogami, Chem. Lett., 44, 995 (2015).