Kyoto Prefectural University
Graduate school of life and environmental sciences
Faculty of environmental sciences
Research topic
Microscopic understanding of chemical reactions occurring in lignocellulose constituents and their related compounds
Lignocellulose is composed mainly of cellulose, hemicelluloses, and lignin.
Cellulose and hemicelluloses, as they are called wood polysaccharides,
are carbohydrate polymers consisting of β-1,4-linked D-glucose, D-mannose, D-xylose monomer units. On the other hand, lignin is an aromatic polymer,
where the C6-C3 units are linked via ether and C-C bonds. Considering the huge amount
of these organic compounds piled on the earth, detailed understanding of
their chemical properties such as chemical structure, reactivity, stability,
etc, is highly important to make deep insights into various natural phenomena.
From the view point of applied science mechanical understanding of various
lignocellulose-related chemical reactions will of great help when their
chemical modification and conversion into chemicals are considered. Here
we introduce several research topics in our laboratory.
Molecular mechanisms in autoxidation of lignin
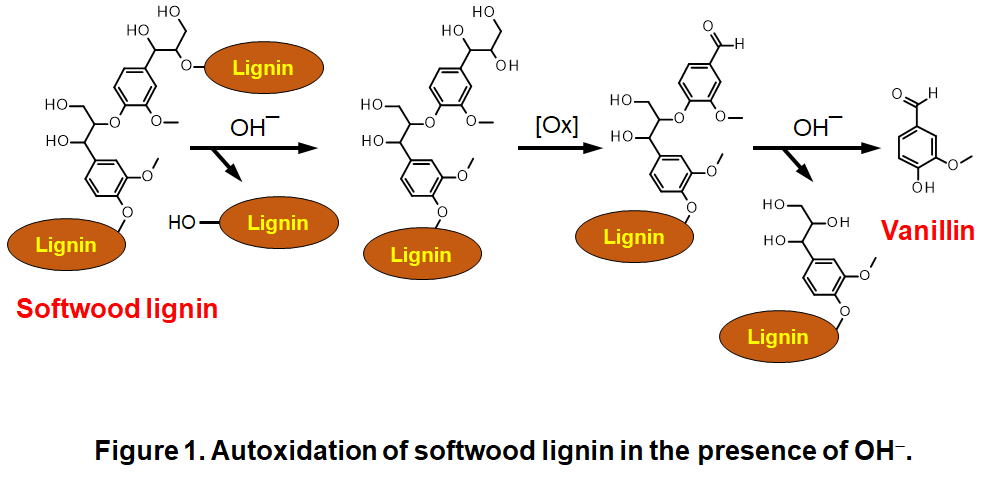
Autoxidation, that is oxidation in atmospheric molecular oxygen, of lignin is a chemical reaction, the mechanical studies of which have long history. Considering the fac that lignin is the second most abundant organic compound on the Earth and molecular oxygen consists about 20 % of the Earth’s atmosphere, autoxidation of lignin has highly important roles in many natural phenomena occurring around us. In addition to this pure scientific motivation, industrial application of the autoxidation makes it feasible to produce various useful chemicals from lignin and molecular oxygen, which is a non-toxic oxidant.Figure 1 shows reaction mechanisms in autoxidation of β-O-4 units, which is a major linkage involved in native lignin, in the presence of OH-. These series of reactions give vanillin (4-hydroxy-3-methoxybenzaldehyde) as a major low molecular weight product. Vanillin is an industrially important compound as a source for several medicines and polymer materials as well as its traditional use as a flavoring agent. We investigate more in-depth of mechanical aspects of this autoxidation to propose methods for efficient controlling of the process.
● Hirano, Y.; Izawa, A.; Hosoya, T.; Miyafuji, H. Degradation mechanism of a lignin model compound during alkaline aerobic oxidation: formation of the vanillin precursor from the β-O-4 middle unit of softwood lignin. React. Chem. Eng. 2022, 7, 1603-1616.
● Hosoya, T.; Yamamoto, K.; Miyafuji, H.; Yamada, T. Selective production of bio-based aromatics by aerobic oxidation of native soft wood lignin in tetrabutylammonium hydroxide. RSC Adv. 2020, 10, 19199-19210.
Mechanistic insights into alkaline nitrobenzene oxidation of lignin
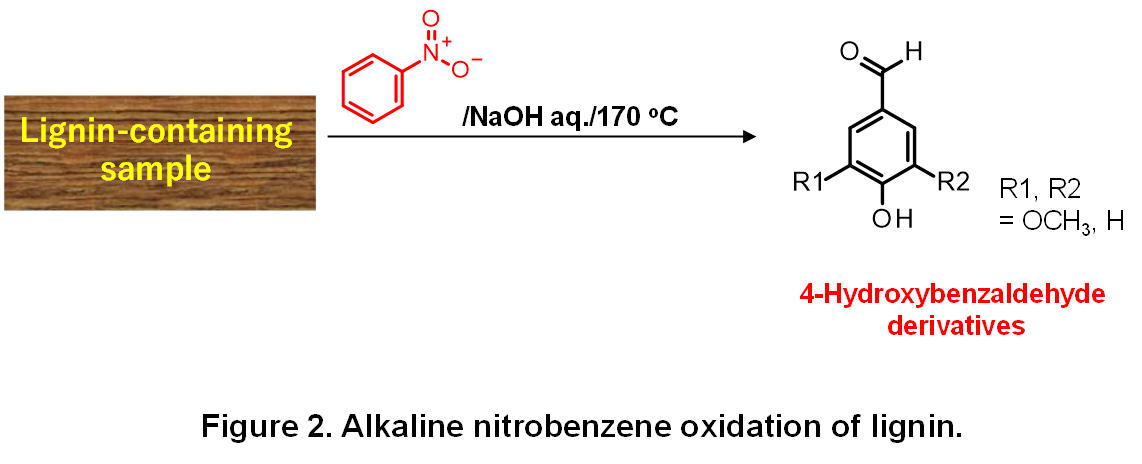
Alkaline nitrobenzene (AN) oxidation is one of the most frequently utilized methods for lignin conversion, where various lignin-containing samples, i.e. wood flour, several technical lignins, isolated-lignins, etc, are oxidized by nitrobenzene in the presence of OH-. AN oxidation usually gives 4-hydroxybenzaldehyes that carry the substitution patterns of the aromatic rings in the original lignin: softwood lignin, for example, gives almost only vanillin and hardwood lignin containing syringyl nuclei form syringaldehyde (4-hydroxy-3,5- dimethoxybenzaldehyde) as well as vanillin. The total yield of these benzaldehydes - when lignin samples with minimum chemical deterioration are utilized - reaches 30~40 wt% of original lignin in AN oxidation, which fact makes AN oxidation one of the most selective chemical conversion methods of lignin. Considering that lignin is a very complex natural polymer involving various types of inter-unit linkages, these high yields are very surprising and enough to make AN oxidation a very powerful analytical method for structural investigation of lignin.However, molecular mechanisms of AN oxidation, in spite of its importance in the relevant research field, have not been fully clarified yet. Detailed information on the mechanisms in AN oxidation will be of great help for further improvement of analytical methods of lignin’s chemical structure that has been attacking attention of many lignin chemists for a century. Also, the molecular mechanisms of AN oxidation are useful for the development of efficient conversion processes of lignin to industrially useful compounds, although industrialization of AN oxidation itself is not realistic because of difficulty in the handling of toxic nitrobenzene. To this end, we are investigating the mechanisms in the benzaldehyde formation and cleavage of various types of covalent bonds in AN oxidation from both sides of experimental and theoretical chemistry.
● Hayashi, T.; Hosoya, T.; Miyafuji, H. Vanillin production pathways in alkaline nitrobenzene oxidation of guaiacylglycerol-β-guaiacyl ether. J. Agric. Food Chem. 2023, 71, 10124-10132.
Quantitative understanding of molecular mechanisms in chemical glycosylation, especially xyloside synthesis
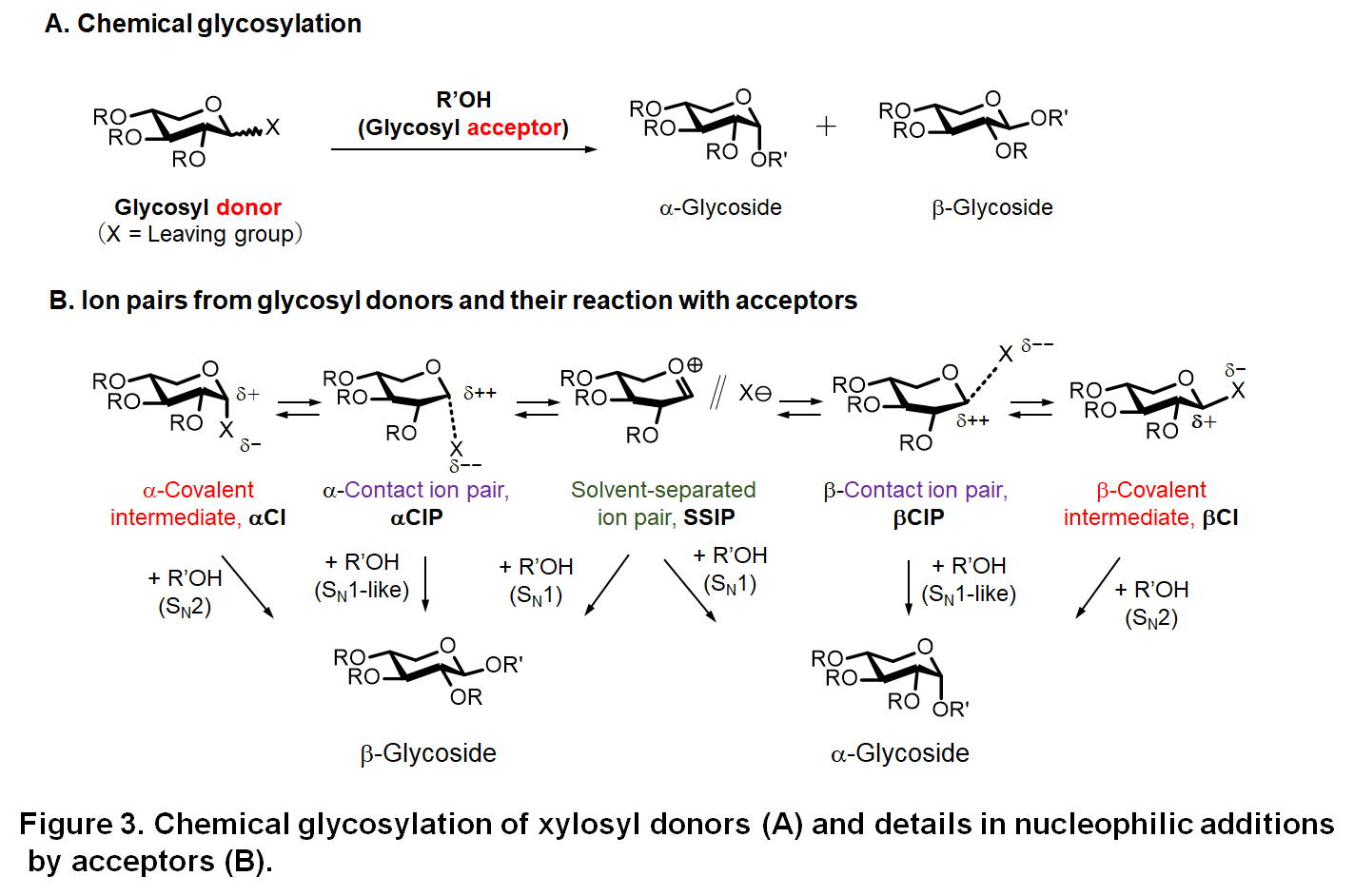
Chemical glycosylation is usually accounted for as a nucleophilic substitution
of a glycosyl donor that carries a leaving group X at its 1-position with
an acceptor (mostly alcohol) to produce a glycosidic linkage, as shown
in Figure 3. This glycosylation is one of the most important reactions
in the field of carbohydrate chemistry and plays pivotal roles in synthesis
of various carbohydrate-based functional molecules. As easily understood
from the fact that wood is composed of polysaccharides that have many glycosidic
linkages, wood chemistry is also closely related to chemical glycosylation.
D-Xylose is a monosaccharide that can be readily produced from woody materials,
especially from hardwoods. Expanding the use of xylose thus leads to promotion
of wood use in human society. To this end we investigate fundamental mechanisms
of chemical glycosylation of xylosyl donors, which underlies production
of various xylose-based useful molecules.
A major issue to be handled in chemical xylosylation (also in general chemical glycosylation) is controlling of the stereochemical selectivity (α/β-selectivity). As shown in Figure 3, glycosylation gives a product mixture consisting of α- and β-isomers, but the target product is usually only one of the isomers. The production of the undesired isomer therefore not only leads to decease in the yield of the target glycoside, but also makes succeeding purification process demanding. Why is such controlling of the stereoselectivity difficult? One of the major reasons is complexity of the mechanisms underlying glycosylation. As show in Figure 3B, a glycosyl donor usually forms an equilibrium mixture consisting of α/β covalent donors (covalent intermediates, CIs) and various ion pairs such as contact ion pairs (CIPs) and solvent separated ion pairs (SSIPs). These nucleophiles, CIs, CIPs, and SSIPs, undergo SN1 and SN2-type reactions, which results in the formation of both α- and β-products.
The controlling of the stereoselectivity eventually requires understanding
of the properties of these species, but elucidation of such properties,
especially detailed structures and reactivity of the ion pairs are not
usually easy, because of their extremely short life time in reaction solution.
Our research group is making basic investigations on molecular mechanisms
of chemical glycosylation, especially xylosylation, combining synthetic
and computational approaches. Quantum chemical calculations facilitate
evaluation of very unstable intermediates and even transition states that
cannot be detected in most of experimental approaches. Our research group
has clarified the energies and the structures of the ion pairs and the
transition states connecting them to the covalent intermediates. More detailed
information is expected to be obtained by further research that follows
these computational results from the experimental side.
● Hosoya, T.; Kosma, P.; Rosenau, T. Effects of a 4,6-diacetal protecting
group on the stability of ion pairs from D-glucopyranosyl and D-mannopyranosyl triflates. Carbohydr. Res. 2015, 411, 64-69.
● Hosoya, T.; Kosma, P.; Rosenau, T. Contact ion pairs and solvent-separated ion pairs from D-mannopyranosyl and D-glucopyranosyl triflates. Carbohydr. Res. 2015, 401, 127-131.
● Hosoya, T.; Takano, T.; Kosma, P.; Rosenau, T. Theoretical foundation
for the presence of oxacarbenium ions in glycoside synthesis. J. Org. Chem. 2014, 79, 7889-7894.