“The need for a new paradigm of self-assembly”
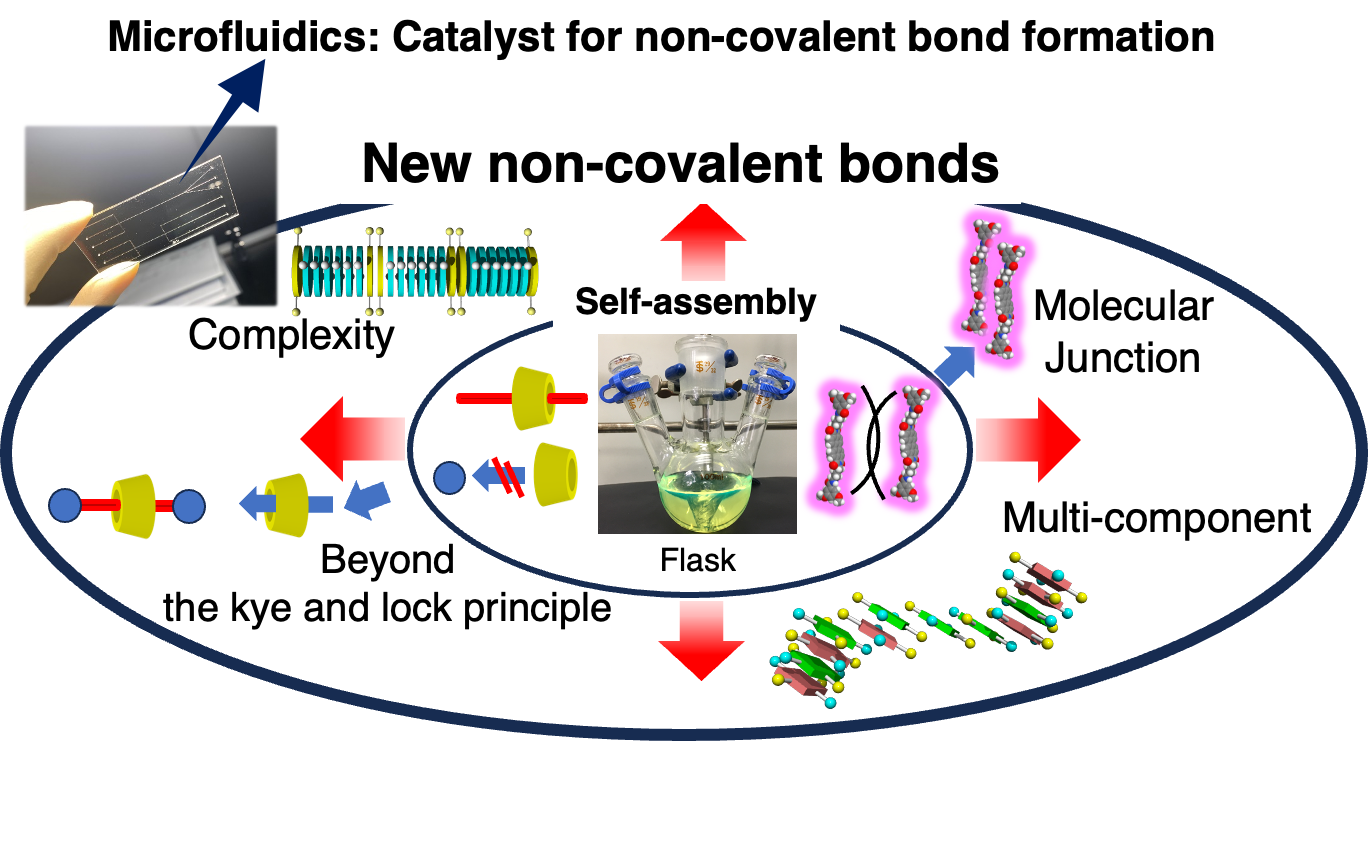
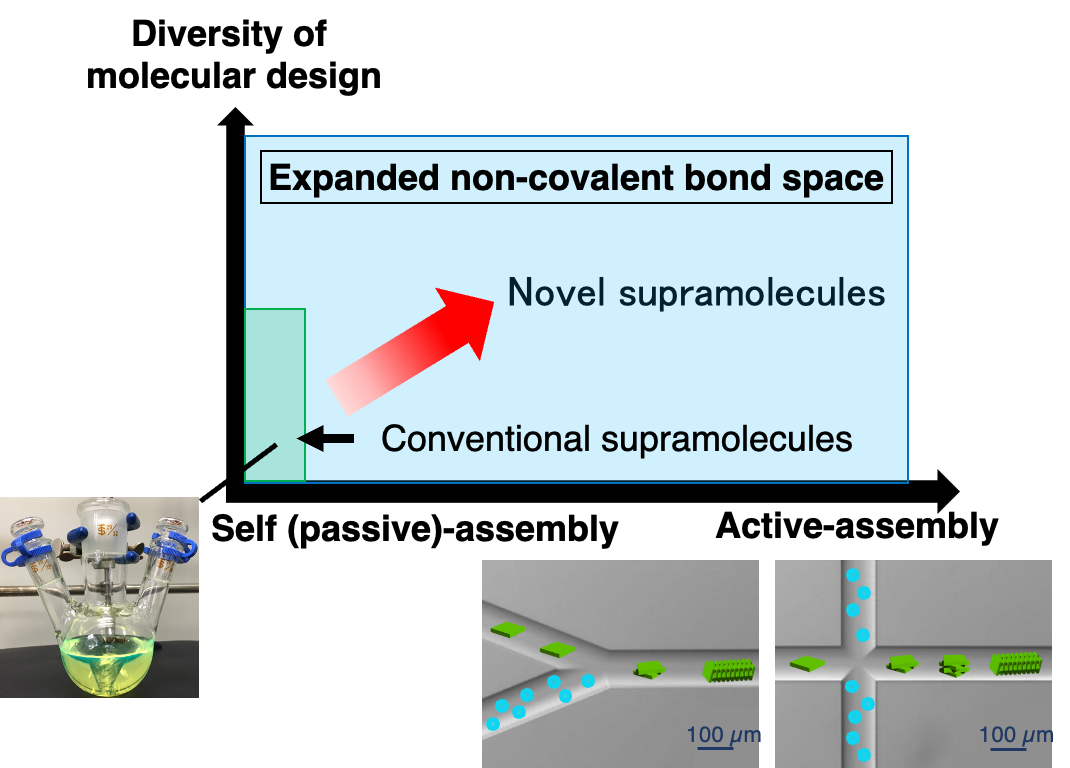
Constructing a versatile self-assembly system will lead to technological innovations in supramolecular chemistry. This would resemble the development of a new reaction or a new catalyst in synthetic organic chemistry. We propose a novel, practical supramolecular assembly system that enable control of intermolecular or interpolymer interactions through precise regulation of a flowing micro-solution.
“Dividing a bulk solution into picoliter-volume solutions“
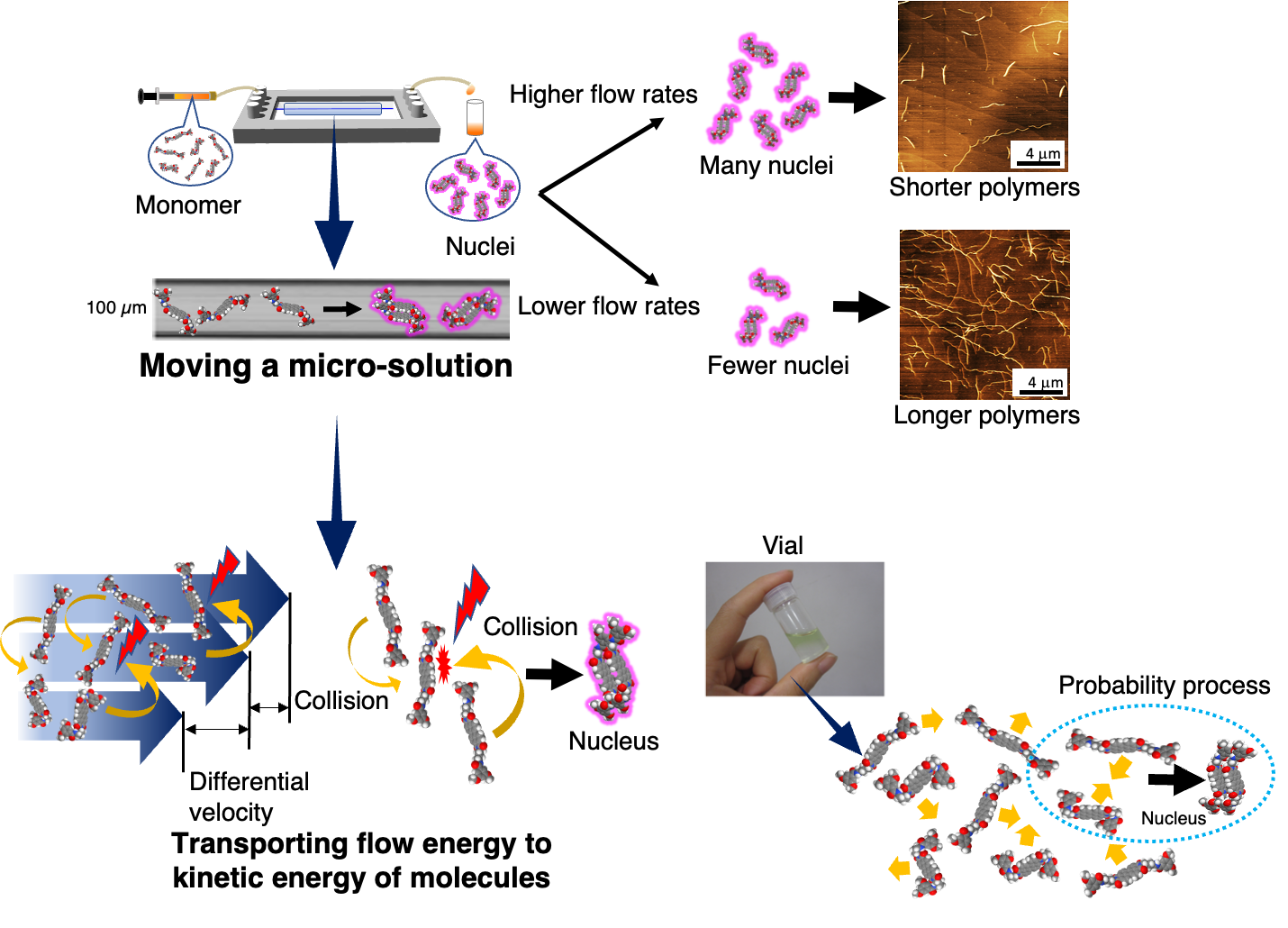
In microflow, self-assembly always occurs during uniform solvent diffusion. In principle, all self-assembly events start simultaneously and finish at the same spatial points; that is, all processes starting with molecules and forming supramolecules and their hierarchical assemblies occur in a synchronized fashion over a molecular scale. This leads to a simultaneous self-assembly of more than thousands of molecules towards monodisperse microstructures.
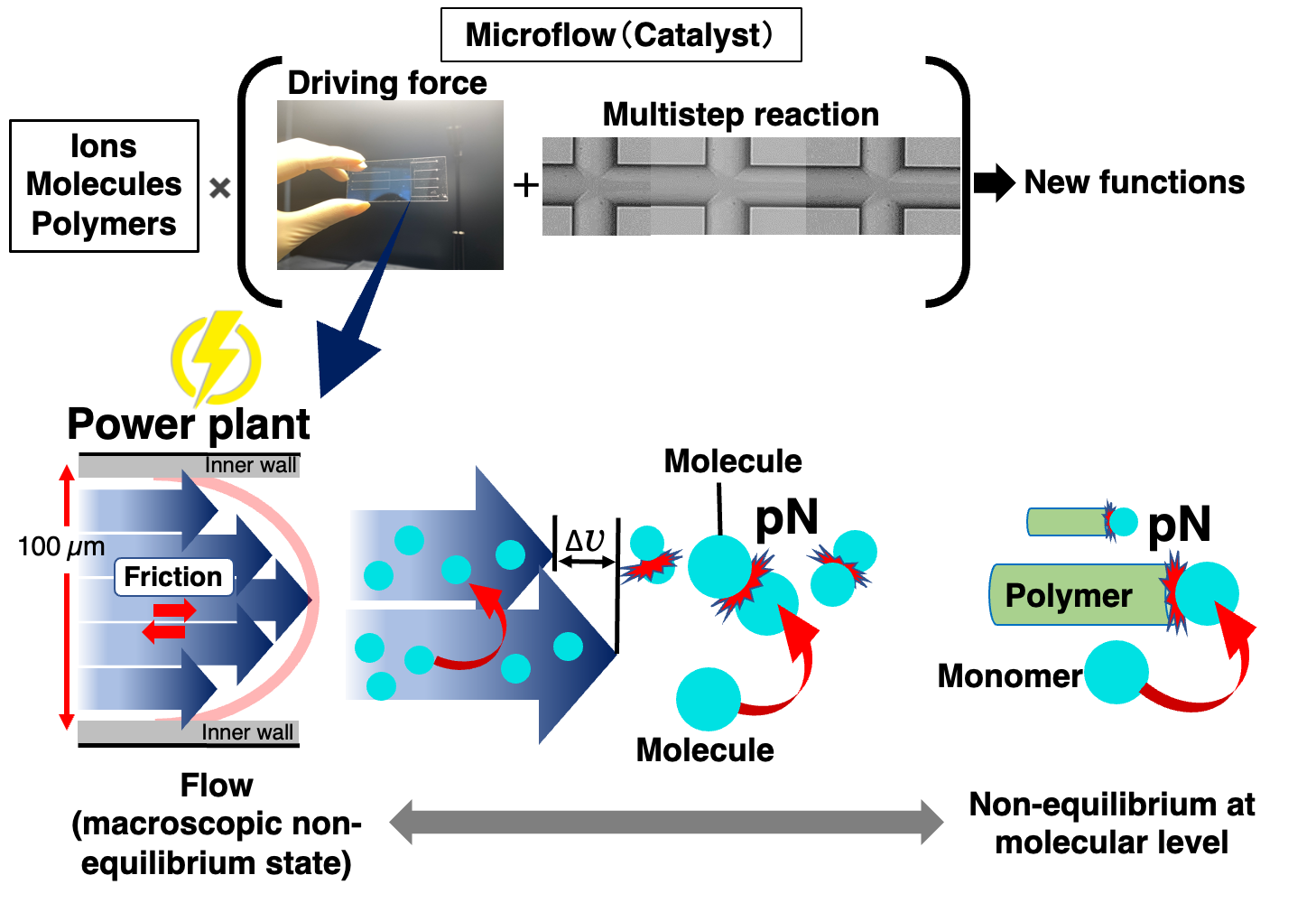
Furthermore, when a solution is moved in a microflow channel, the internal friction is effectively converted into the kinetic energy of the molecules thus to drive the formation of non-covalent bonds. The collision energy induced in the microflow channel is quite low and hence insufficient to cause chemical reactions. However, this energy is sufficient to induce weak non-covalent bonds. Upon just moving a solution in a microflow channel, conventional self-assembly becomes a more positive process, leading to the creation of new supramolecular structures.
“Development of a catalyst in supramolecular chemistry”
Microfluidics itself is a low Reynolds number solution. Thereby, the motion of supramolecules in it is also under a strong influence of viscosity. In case the supramolecular polymerization, the supramolecular polymers are oriented and move linearly in freely diffusion monomers. This contrasting motion between the supramolecular polymer and monomers resulted in anisotropic supramolecular polymerization. Microflow accelerates non-covalent bond formation with favorable collision, energetic and steric effects. From the mechanism, it can be said that a microflow channel serves as sort of catalyst for supramolecular polymerization.
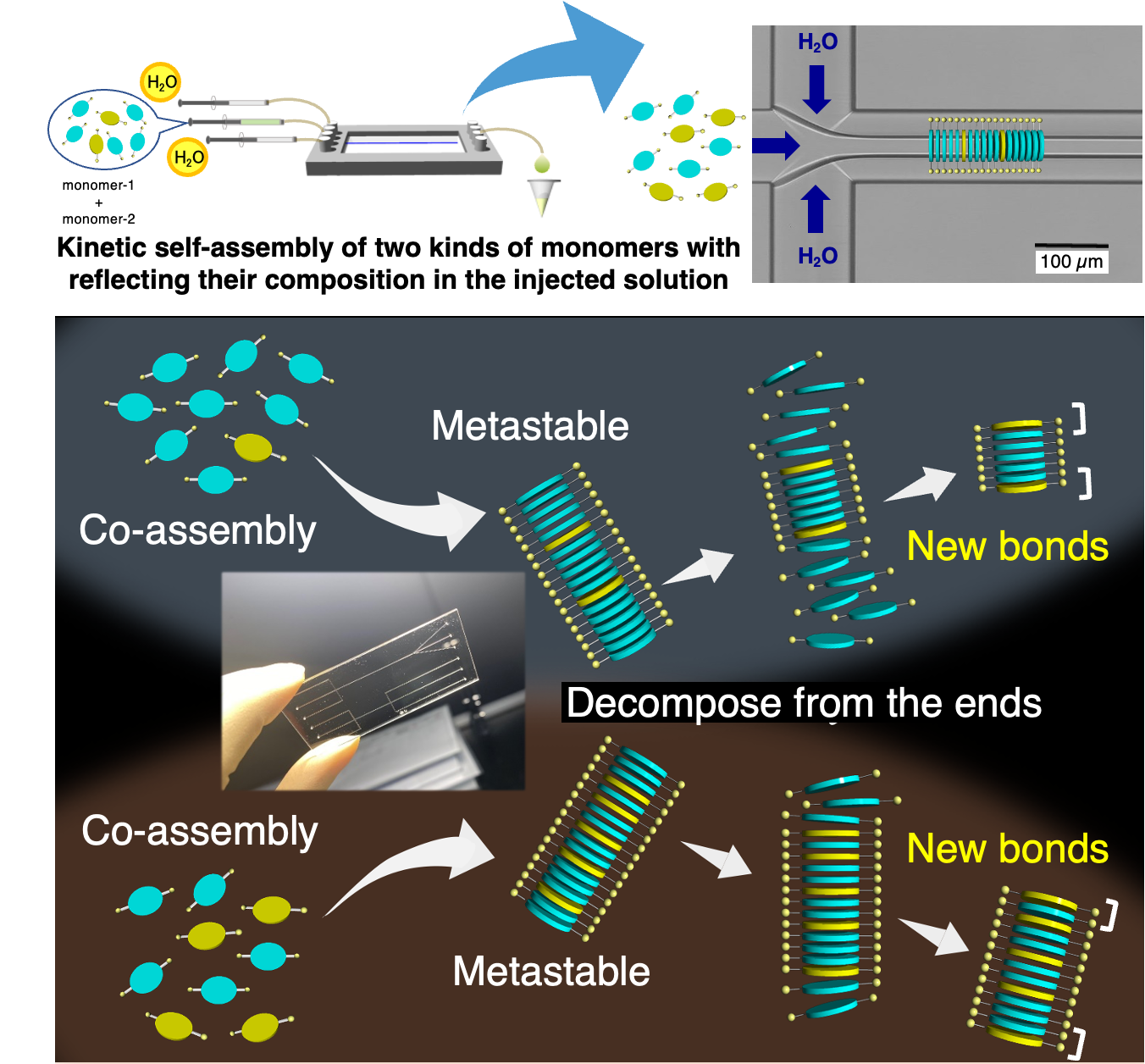
“Supramolecular co-assembly reflecting the monomer composition in the injected solution”
One major concern in supramolecular chemistry is how to place different intermolecular interactions in a desired position. Kinetic co-assembly of two kinds of molecules having different interaction moieties realized in a microflow channel. In the created metastable supramolecular co-polymers, relatively stronger interactions were regularly inserted as stabilizing wedges. It was found that decomposition of the supramolecular co-polymers from the ends was suppressed at the wedges, leading to the creation of discrete 1D structures with capped ends.
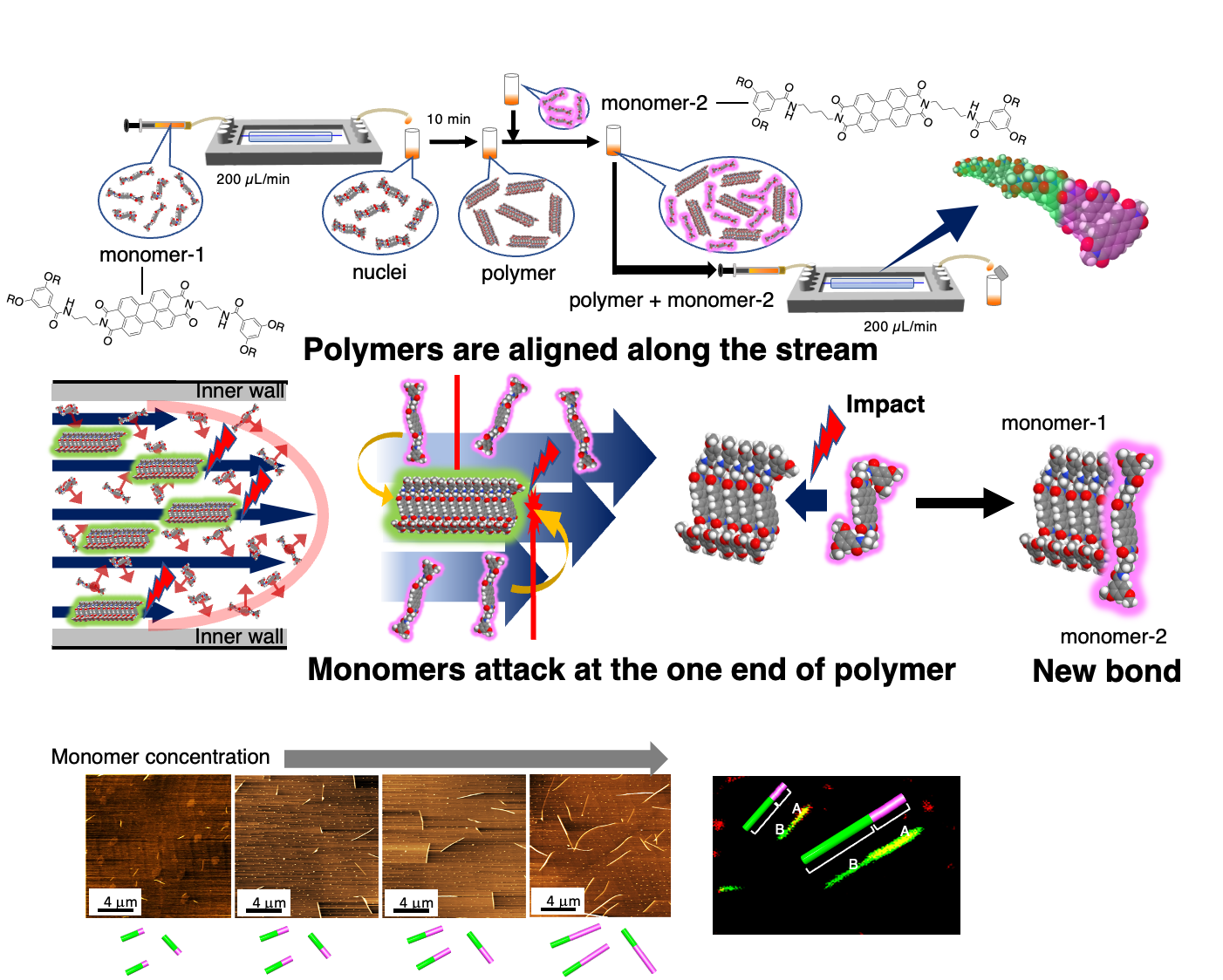
“Spatial control over self-assembly dynamics:Direct correlation between self-assembly dynamics and flow distance”
In principle, self-assembly dynamics can be controlled along a channel. In the case of diverse self-assemblies, such as supramolecular polymers, the self-assembly number (molecular weight) can be regulated by the flow distance. Microflow provided an epoch-making strategy to control the length (molecular weight) of supramolecular polymers. In general case, polymerization rates were able to be estimated by evaluation of the time required for termination to be several tens of milliseconds.

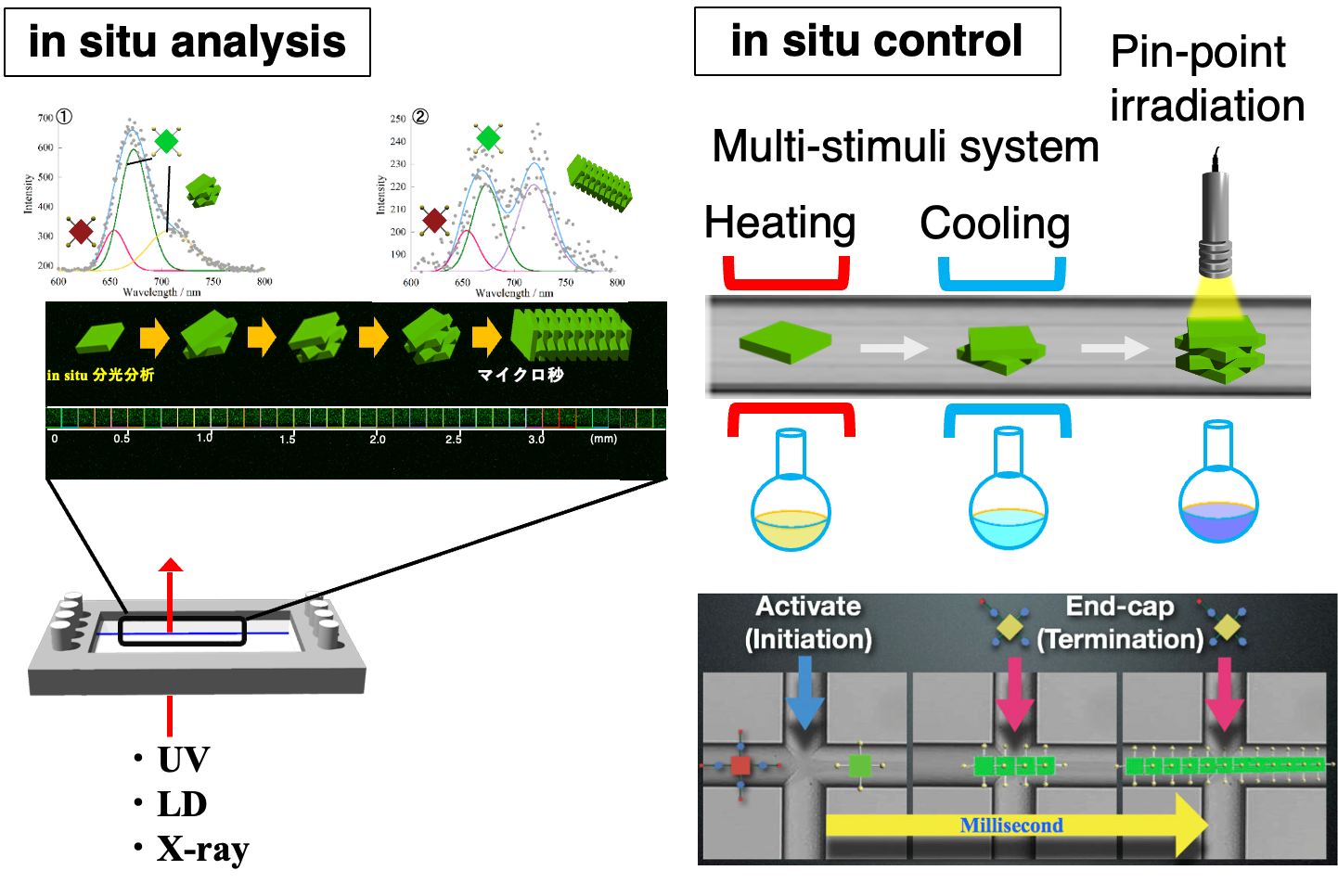
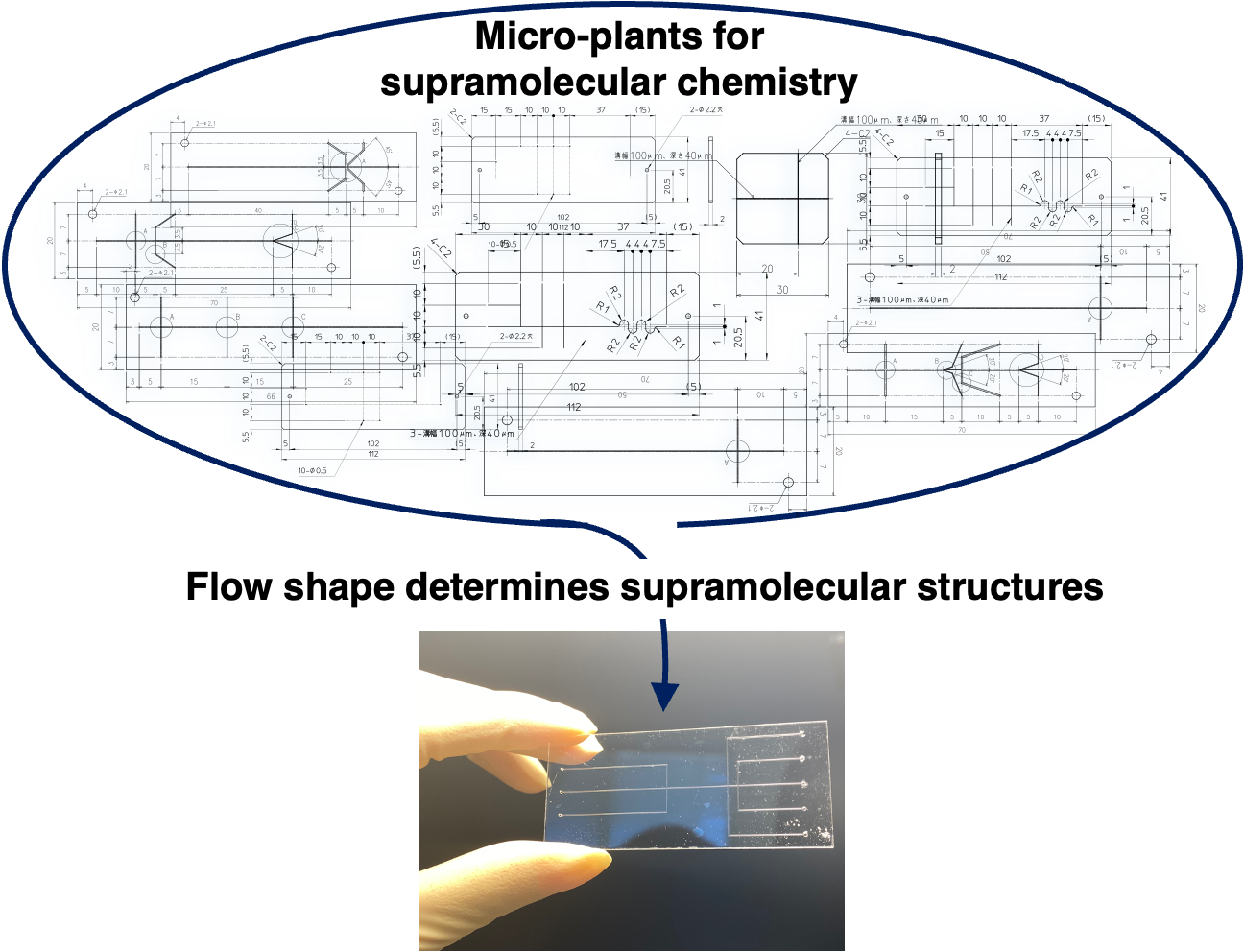
“Development of a micro-plant for supramolecular chemistry”
In supramolecular chemistry, no standard protocol exists for the purification and recovery of unreacted molecules. This is a huge drawback in terms of the development of practical self-assembly system through multi-step reactions.
Recent development of microfabrication technologies enables us to fabricate a complicated microchannel for supramolecular chemistry. When designing an appropriate microflow channel, self-assembling species, as ions, molecules, and polymers, can be transported systematically to the desired point and meet at appropriate times. Furthermore, purification and recovery processes can be combined into a single flow system. This system will act as a practical micro-plant for the non-covalent synthesis.